


Pharmacodynamics of once-daily amikacin in various combinations with cefepime, aztreonam, and ceftazidime against Pseudomonas aeruginosa in an in vitro infection model.Clinical evaluation of this combination is warranted. This positive interaction appears to be due in part to the ability of aztreonam to protect cefepime from extracellular cephalosporinase inactivation. aeruginosa producing increased levels of cephalosporinase. The results of these studies demonstrate that aztreonam can enhance the antibacterial activity of cefepime against derepressed mutants of P. aeruginosa strains producing increased levels of cephalosporinase demonstrated that the enhanced pharmacodynamics of cefepime-aztreonam were not unique to the isogenic panel. Further pharmacodynamic studies with five other P.
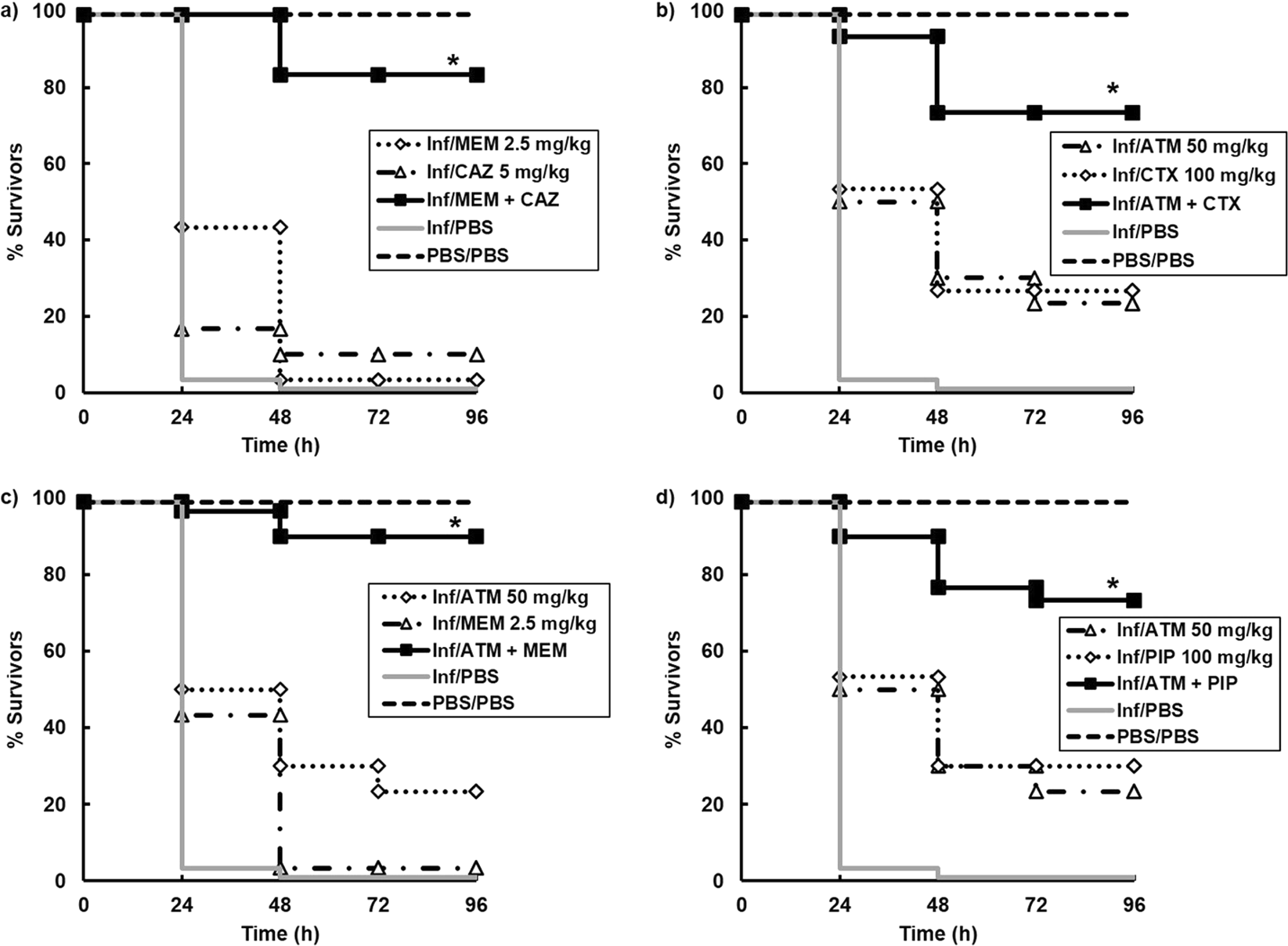
In contrast to cefepime-aztreonam, the pharmacodynamics of ceftazidime-aztreonam were not enhanced over those of aztreonam alone. Hydrolysis and bioassay studies demonstrated that aztreonam was inhibiting the extracellular cephalosporinase that had accumulated and was thus protecting cefepime in the extracellular environment. Against PD and FD strains, the antibacterial activity of cefepime-aztreonam was significantly enhanced over that of each drug alone, with 3.5 logs of killing by 24 h. Against WT strains, the cefepime-aztreonam combination was the most active regimen, but viable counts at 24 h were only 1 log below those in cefepime-treated cultures. In studies with cefepime and cefepime-aztreonam against the PD strain, samples were also filter sterilized, assayed for active cefepime, and assayed for nitrocefin hydrolysis activity before and after overnight dialysis. Logarithmic-phase cultures were exposed to peak concentrations achieved in serum with 1- or 2-g intravenous doses, elimination pharmacokinetics were simulated, and viable bacterial counts were measured over three 8-h dosing intervals. N2 - An in vitro pharmacokinetic model was used to determine if aztreonam could enhance the pharmacodynamics of cefepime or ceftazidime against an isogenic panel of Pseudomonas aeruginosa 164, including wild-type (WT), partially derepressed (PD), and fully derepressed (FD) phenotypes. JF - Antimicrobial agents and chemotherapy T1 - Cefepime-aztreonam: a unique double beta-lactam combination for Pseudomonas aeruginosa. An in vitro pharmacokinetic model was used to determine if aztreonam could enhance the pharmacodynamics of cefepime or ceftazidime against an isogenic panel of Pseudomonas aeruginosa 164, including wild-type (WT), partially derepressed (PD), and fully derepressed (FD) phenotypes.


 0 kommentar(er)
0 kommentar(er)
